SUZHOU and SHANGHAI China, Aug 26, 2025 -Forlong Biotechnology, a clinical stage biotech dedicated to developing next-generation cytokine therapeutics, today announces that the first patient has been dosed in China on August 23, 2024, at Fudan University Shanghai Cancer Center, in the ongoing Phase I/II trial (CTR20242361) evaluating FL115 monotherapy and in combination with Bacillus Calmette-Guérin (BCG) for non-muscle-invasive bladder cancer (NMIBC).
The trial is an open-label, multicenter, Phase I/II dose-escalation and expansion study designed to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of FL115 as monotherapy and in combination with BCG in patients with NMIBC. The study is led by Professor Dingwei Ye at Fudan University Shanghai Cancer Center.
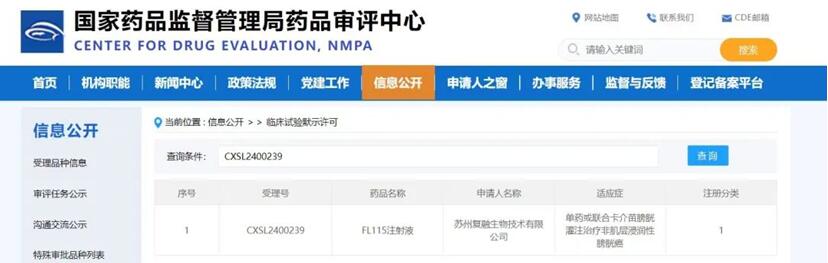
On Jun 24 2024, this trial was approved CDE(IND application:CXSL2400239)as the second IND approval Forlong Biotech has obtained this year, underscoring the team’s outstanding R&D capability and high-execution efficiency.
About FL115
FL115 is a next-generation, long-acting IL-15/IL-15Rα monomeric Fc-fusion protein wholly developed by Forlong Biotech via Fbody platform. Its unique single-arm structure delivers antibody-like yields, enhanced tumor penetration, and an exemplary safety profile. Pre-clinical studies demonstrate FL115 has significantly wider therapeutic index than comparator IL-15 agents, enabling efficacious dosing at markedly higher exposure levels.
About Forlong Biotechnology
Forlong Biotechnology is a clinical stage biotechnology company dedicated to developing next-generation cytokine therapeutics. Based on synthetic immunology, the company has established four core technology platforms, committed to overcoming the challenges of developing cytokine drugs.
Focusing on tumor immunology, Forlong develops engineering therapeutic cytokine combinations that act at distinct steps of the cancer–immunity cycle to treat cancer.



 苏公网安备32050502011888号
苏公网安备32050502011888号