The lead candidate FL115 is an interleukin-15 (IL-15) superagonist, which can stimulate natural killer cells (NK cells) and specific memory T cells, and has a complementary and synergistic effect with existing T cell-driven immunotherapies such as PD-1 inhibitors. FL115 is in multiple Phase I clinical studies in China and the United States, showing robust safety profile and initial clinical efficacy in patients with advanced solid tumors. It is the only IL-15 super agonist to achieve partial response (PR) as a monotherapy and has the potential to be the best-in-class globally, currently being advanced to combo therapy with PD-1 antibodies in clinic. FL115 monotherapy and BCG combo has shown robust safety profile and initial clinical efficacy in NMIBC, currently being advanced to Phase II.
FL116 is fusion protein of a PD-1 antibody and an engineered interleukin-18 (IL-18), which can stimulate effector T cells with potential to turn a potential "cold" tumor into a "hot" tumor. It has demonstrated significant tumor-killing ability in preclinical studies of several tumor models that are resistant to immune checkpoint inhibitors (CPI).
- Pipeline
- Indication
-
ResearchIND-enablingINDPhase IPhase II
- Partners
| FL115 (IL-15 super agonist) |
Mono (IV) | Solid Tumors | ||
| Combo with PD-(L)1 (IV) | NSCLC;Melanoma; Other solid tumors |
|||
| Combo with PD-(L)1 (SQ) | Solid Tumors | |||
| Combo with BCG (Intravesical Instillation) |
NMIBC |
FL115 Introduction:
IL -15
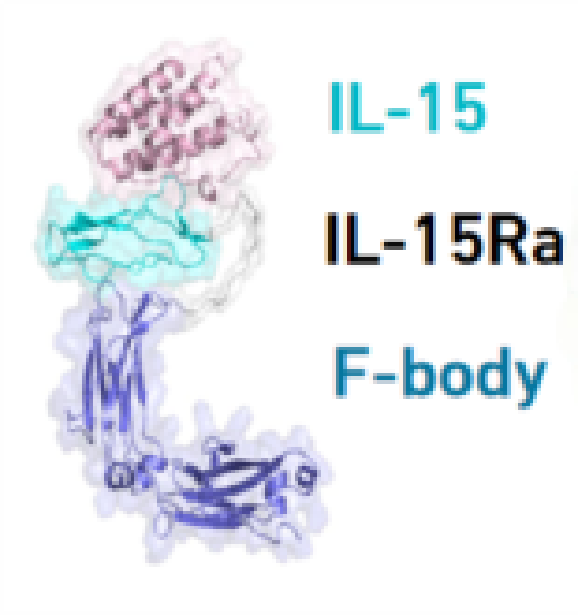 Interleukin-15 (IL-15) is a rare cytokine that can selectively activate natural killer cells (NK cells) while also stimulating specific memory T cells. Existing tumor immunotherapy drugs, such as immune checkpoint inhibitors, primarily focus on activating T cells. IL-15 offers a new mechanism for tumor killing and plays a significant role in tumor immunotherapy.
Interleukin-15 (IL-15) is a rare cytokine that can selectively activate natural killer cells (NK cells) while also stimulating specific memory T cells. Existing tumor immunotherapy drugs, such as immune checkpoint inhibitors, primarily focus on activating T cells. IL-15 offers a new mechanism for tumor killing and plays a significant role in tumor immunotherapy.
In April 2024, the world's first IL-15 super agonist, Anktiva®, was approved for marketing in the United States. Its combination therapy with PD-1 monoclonal antibodies has advanced to Phase 3 clinical trials. Meanwhile, companies such as Nektar Therapeutics, Sanofi, and BeiGene are also developing various types of IL-15 agonists, which are currently in Phase 1/2 clinical trials.
FL115
Clinical translation of of IL-15 faces significant challenges, such as its small molecular weight, short half-life, and functionality that depends on binding to receptors on different immune cells. Forlong Biotech has developed FL115, a fusion protein composed of IL-15, IL-15Rα, and Fbody, through its own platform technology, significantly expanding the therapeutic window. FL115 has demonstrated superior safety and tumor-killing ability to Anktiva® in multiple preclinical models. Currently, multiple clinical trials of FL115 are ongoing in China and the United States. As a monotherapy, it has shown good safety and initial clinical efficacy in patients with advanced solid tumors. It is the only IL-15 super agonist to achieve partial response (PR) as a monotherapy and has the potential to be the best-in-class globally.
IL-15 has been hindered by its low molecular weight and short half-life. Leveraging proprietary FBody platform, Forlong Biotech engineered a single-arm IL-15 fusion protein that markedly extends half-life and widens the therapeutic window. FL115 is currently in multiple Phase I clinical studies in China and the United States, showing robust safety profile and initial clinical efficacy in patients with advanced solid tumors. It is the only IL-15 super agonist to achieve partial response (PR) as a monotherapy and has the potential to be the best-in-class globally, currently being advanced to combo therapy with PD-1 antibodies in clinic. FL115 monotherapy and BCG combo has shown robust safety profile and initial clinical efficacy in NMIBC, currently being advanced to Phase II.
-
FL116
(anti-PD-1/lL-18 fusion protein) - Solid Tumors
FL116 Introduction:
IL-18
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that activates effector T cells and NK cells, and induces the secretion of IFN-γ, thereby transforming "cold" tumors into "hot" tumors. Therefore, it is considered an ideal candidate molecule for cancer immunotherapy. However, the presence of IL-18 binding protein (IL-18BP) in the tumor microenvironment can antagonize the binding of IL-18 to its receptor, impeding its function. This has become a major bottleneck in the development of IL-18 agonists. Additionally, CMC issues limit the drug development of wild-type IL-18. Currently, the most advanced IL-18 agonist globally is in Phase I clinical trials.
Forlong IL-18 assets
Leveraging its Intelligent Biomolecular Discovery Platform and Syntokine® Synthetic Cytokine Platform, Forlong Biotech has developed a portfolio of IL-18 mutants with different preference of IL-18 receptor affinities vs IL-18 binding protein, as well as better drug-like properties.These IL-18 mutant molecules can be combined with other proteins (such as monoclonal antibodies) to develop multiple product pipelines.
- FL116 is an anti-PD-1/lL-18 fusion protein that can significantly enhance binding to T cells through cis- action, activating effector T cell functions. It has demonstrated potent tumor-killing capability in preclinical studies of several tumor models that are resistant to immune checkpoint inhibitors (CPI).
- FL117 is a tumor cell-targeted IL-18 fusion protein that can selectively recruit and activate T cells in the tumor microenvironment, mediating potent anti-tumor killing ability.
- FL118 is a non-targeted IL-18 fusion protein that can systemically activate effector T cells, and hold promise when combined with other immunotherapies across multiple indications.
-
FL117
(TAA targeted IL-18) - Solid Tumors
FL117 Introduction:
IL-18
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that activates effector T cells and NK cells, and induces the secretion of IFN-γ, thereby transforming "cold" tumors into "hot" tumors. Therefore, it is considered an ideal candidate molecule for cancer immunotherapy. However, the presence of IL-18 binding protein (IL-18BP) in the tumor microenvironment can antagonize the binding of IL-18 to its receptor, impeding its function. This has become a major bottleneck in the development of IL-18 agonists. Additionally, CMC issues limit the drug development of wild-type IL-18. Currently, the most advanced IL-18 agonist globally is in Phase I clinical trials.
Forlong IL-18 assets
Leveraging its Intelligent Biomolecular Discovery Platform and Syntokine® Synthetic Cytokine Platform, Forlong Biotech has developed a portfolio of IL-18 mutants with different preference of IL-18 receptor affinities vs IL-18 binding protein, as well as better drug-like properties.These IL-18 mutant molecules can be combined with other proteins (such as monoclonal antibodies) to develop multiple product pipelines.
- FL116 is an anti-PD-1/lL-18 fusion protein that can significantly enhance binding to T cells through cis- action, activating effector T cell functions. It has demonstrated potent tumor-killing capability in preclinical studies of several tumor models that are resistant to immune checkpoint inhibitors (CPI).
- FL117 is a tumor cell-targeted IL-18 fusion protein that can selectively recruit and activate T cells in the tumor microenvironment, mediating potent anti-tumor killing ability.
- FL118 is a non-targeted IL-18 fusion protein that can systemically activate effector T cells, and hold promise when combined with other immunotherapies across multiple indications.
-
FL118
(non-targeted IL-18) - Solid Tumors
FL118 Introduction:
IL-18
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that activates effector T cells and NK cells, and induces the secretion of IFN-γ, thereby transforming "cold" tumors into "hot" tumors. Therefore, it is considered an ideal candidate molecule for cancer immunotherapy. However, the presence of IL-18 binding protein (IL-18BP) in the tumor microenvironment can antagonize the binding of IL-18 to its receptor, impeding its function. This has become a major bottleneck in the development of IL-18 agonists. Additionally, CMC issues limit the drug development of wild-type IL-18. Currently, the most advanced IL-18 agonist globally is in Phase I clinical trials.
Forlong IL-18 assets
Leveraging its Intelligent Biomolecular Discovery Platform and Syntokine® Synthetic Cytokine Platform, Forlong Biotech has developed a portfolio of IL-18 mutants with different preference of IL-18 receptor affinities vs IL-18 binding protein, as well as better drug-like properties.These IL-18 mutant molecules can be combined with other proteins (such as monoclonal antibodies) to develop multiple product pipelines.
- FL116 is an anti-PD-1/lL-18 fusion protein that can significantly enhance binding to T cells through cis- action, activating effector T cell functions. It has demonstrated potent tumor-killing capability in preclinical studies of several tumor models that are resistant to immune checkpoint inhibitors (CPI).
- FL117 is a tumor cell-targeted IL-18 fusion protein that can selectively recruit and activate T cells in the tumor microenvironment, mediating potent anti-tumor killing ability.
- FL118 is a non-targeted IL-18 fusion protein that can systemically activate effector T cells, and hold promise when combined with other immunotherapies across multiple indications.
- FL301
- Solid Tumors
-
FL401
(Collaboration Program) - Solid Tumors

- Other Cytokines
- Multiple diseases



 苏公网安备32050502011888号
苏公网安备32050502011888号